oSATCo App Development:
Data Collection
A placeholder description and if this description had more words
how would that look?

Objectives
Data was collected for the purpose of:
SATCo
- Cross sectional study
- Objective: train neural network on a wide range of children
- Longitudinal study
- Objective: check repeatability and reliability of oSATCo results
Functional assessments
- Objective: compare outcomes from SATCo and functional assessments
Project objectives:
1. Acquisition of a substantial cross-sectional dataset demonstrating the range of problems of trunk control in children with CP.
2. Acquisition of a cross-sectional dataset demonstrating a sample of problems of trunk control in children with SMA1.
3. Acquisition of a longitudinal dataset of children quantifying trunk control status to provide sufficient data to plan appropriately powered clinical trials using the tool.
4.Acquisition of an exploratory cross-sectional dataset defining the range of problems of trunk control in children with neuromuscular disorders including congenital myopathy, congenital muscular dystrophy, SMA type 2 and Duchennemuscular dystrophy to aid understanding of the natural progression of these conditions and enhance management.
Eligibility criteria
Inclusion criteria
- Children with
- clinical diagnosis of CP, 2-16 years old.
- genetically confirmed SMA1 diagnosis, 3 months-16 years old.
- genetically confirmed diagnosis of NMD, 2-16 years old
- Parents/guardians with capacity to give consent.
Exclusion criteria
- SMA1 children who are on mechanical ventilation ≥16 hours per day or deemed clinically unfit to participate.
- Both parents/guardians do not have sufficient understanding of English to give consent.
- Children who pose a physical risk to themselves or the research team.

Patient Identification, Recruitment, and Consent
Participant identification by:
- CP: Community paediatric physiotherapists
- SMA and NMD: Neuromuscular consultants and physiotherapists.
⇣
Parent/Guardian contact with:
- Letter of invitation sent by the care team
- Information packs given in routine clinical appointment
⇣
Once parent/guardian demonstrated interested:
- Data collection date and room arrangement between parent/guardian, care team, and data collection team
- Informed Consent signed before data collection
Baseline Data
We collected the following baseline data from the participants:
- Age
- Body height or length
- Body weight
- Medical record
- Main diagnosis
- Relevant medical history
- Classification, for CP: GMFCS and clinical type
- SATCo
- Functional assessment
- CP
- GMFM-Research Item Set (carried out by research team on site)
- SMA
- SMA Reach (provided by clinical staff from sites)
- NMD
- CHOP-INTEND, NSAA, etc (provided by clinical staff from sites)
- CP

What was involved in the data collection process?
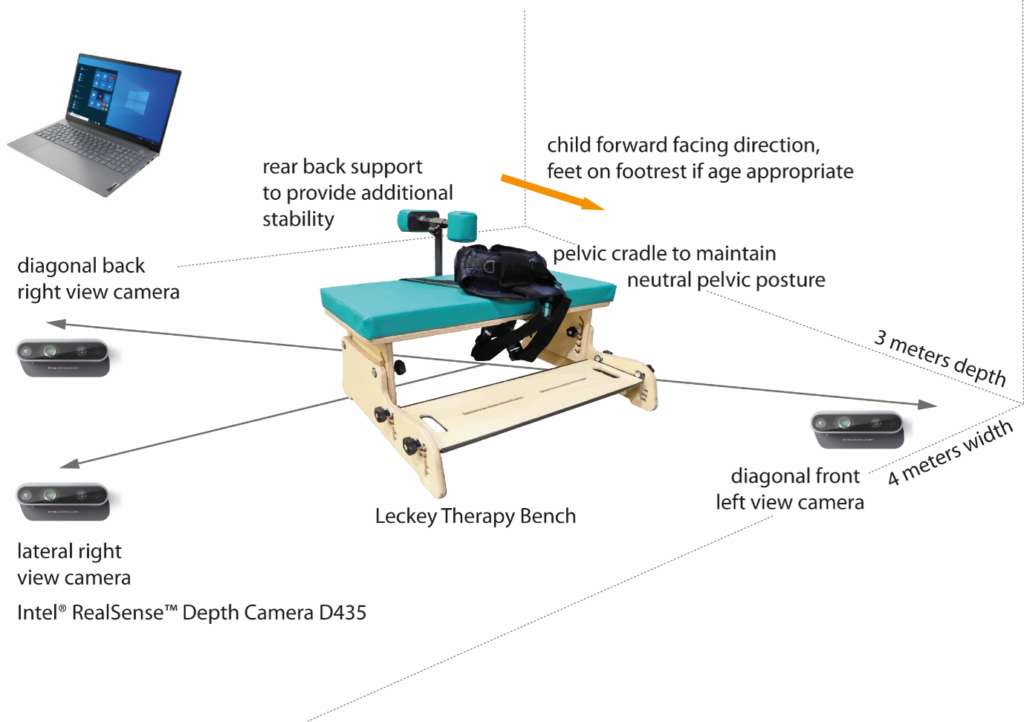
- Location
- hospitals, clinics, schools, community centers, MMU
- Travel
- rental cars, trains, and taxi
- Equipment set up
- duration 30 mins
- mobile set up: laptop, camera, tripods, bench, harnesses, toys
- no markers are attached to the child
- SATCo
- duration 10-50 mins
- Functional assessment
- CP only: GMFM, duration 10-30 mins
How far did we travel for the project?
We travelled across England’s Northwest, West Midlands, and Yorkshire and the Humber regions to collect data.
| External Data Collection Sessions: | > 100 |
| Miles Travelled: | > 6000 miles |
| Partnered Sites |
| Manchester University NHS Foundation Trust |
| Alder Hey Children’s NHS Foundation Trust |
| Lancashire Teaching Hospitals NHS Foundation Trust |
| The Leeds Teaching Hospitals NHS Foundation Trust |
| The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust |
| Sandwell and West Birmingham NHS Trust |
| University Hospitals Birmingham NHS Foundation Trust |
| Seashell |

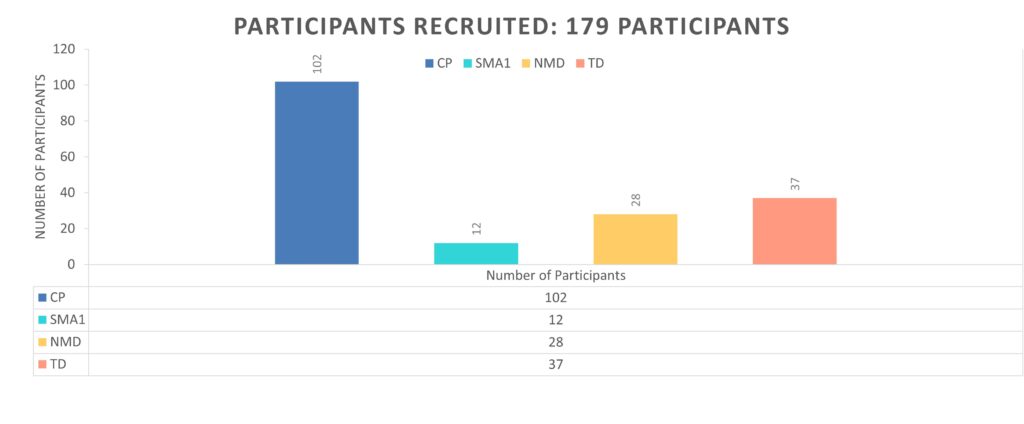
We recruited 179 participants for the cross-sectional study.
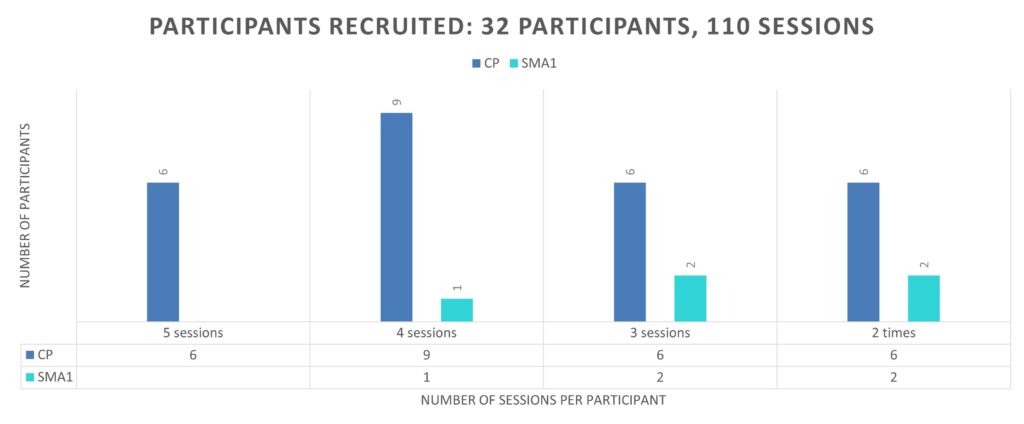
We recruited 32 participants for the longitudinal study. Each participant took part in two to five sessions.
What barriers were faced during the data collection process?
- Cancelled Appointments
- Illnesses/hospital admissions
- Space restraints (community)
Special Acknowledgements
- Family of participants
- All involved staff members




